PRONEURA®
ReacX’s proprietary long-term, drug delivery platform provides continuous drug release and non-fluctuating medication levels over a period of six months or longer depending on drug characteristics. ProNeura products are subdermal implants that are intended for treating chronic diseases for which the maintenance of stable medication levels could offer advantages over other routes of administration.
The first product based on ProNeura technology was Probuphine® (buprenorphine) implant. Probuphine was approved in the United States, Canada and the European Union, or EU, for the maintenance treatment of opioid use disorder in clinically stable patients taking 8 mg or less a day of oral buprenorphine, In October 2020, this product was withdrawn from the market as the company marketing this product decided to wind down their commercialization activities. ReacX Pharmaceuticals has acquired Probuphine and the Proneura technology, and is looking forward to relaunching Probuphine and to continuing the development of ProNeura technology-based products for other therapeutic indications.
Home » Technology
OVERVIEW
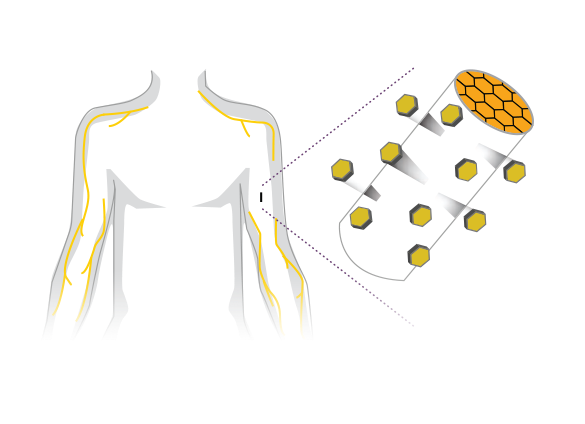
ProNeura® offers continuous drug delivery and consists of a small, semi-rigid, flexible implant made from a mixture of ethylene-vinyl acetate (EVA) and a drug substance. The resulting product is a solid matrix implant that is placed subcutaneously, normally in the inner part of the upper arm in a simple office procedure, and is removed in a similar manner at the end of the treatment period.
This continuous drug delivery technology was developed to address the need for a simple, practical method to potentially provide continuous drug administration on an outpatient basis over extended periods of six months or longer.
MAKING PRODUCTS WITH PRONEURA®
The drug substance that is part of the ProNeura implant is slowly released subdermally into the patient’s body at a continuous constant rate, through the process of dissolution and diffusion through pores in the implant’s matrix. The outcome is a stable level of medication in the blood for the duration of the treatment and bioavailability, similar to that seen with intravenous administration. Such long-term, linear release characteristics are generally desirable, as this avoids peak-and-trough level dosing that poses problems in the treatment of several chronic diseases.
HOW PRONEURA® IS MADE
To manufacture the ProNeura implant, EVA polymer is blended with the medication and extruded. This is then cut to required size to create implants, within which the medication is uniformly distributed throughout the EVA matrix. In some cases, multiple concentric layers of EVA and medication(s) may be employed to facilitate the desired end product characteristics.

COMPETITIVE ADVANTAGE OF PRONEURA®
By providing continuous, stable blood levels of medication, the ProNeura subdermal implant has the potential to offer several significant benefits to patients compared with other routes of administration. In addition to potential safety, efficacy and tolerability advantages resulting from long-term, non-fluctuating drug exposure, ProNeura would be implanted in a short, outpatient procedure once every six months or longer, making it more convenient for patients and enhancing compliance.
Product Pipeline
Probuphine® (buprenorphine) Implant
for the treatment of opioid addiction
Product Pipeline
NALMEFENE IMPLANT
for the prevention of relapse in OUD patients following detoxification from opioids